Refex's electrodes provide stable, accurate readings in the most taxing environments.
At the heart of every Refex electrode is a unique nonporous reference system that delivers high quality performance, stable readings and accurate pH/ORP measurements. Refex sensors last many times longer than conventional electrodes with porous junction reference cells, require minimal maintenance, and exhibit minimal drift.
Refex sensors are available in many industry standard sizes, and can easily be retrofitted for existing systems and housings. For highly specific applications, South Fork Industries will work with you to design a measurement solution that meetings your specifications.
What are pH and ORP electrodes?
Electrodes are electrochemical sensors used to measure pH or ORP (oxidation reduction potential) in industrial process applications. From clean water to sewage sludge, from biopharmaceuticals to petroleum, they can be put to work everywhere.
It’s most likely that the type of electrode you’ve come across in your work is a glass electrode, as these are the most common. Don’t be deceived by the simple design of this humble electrode—there’s a lot going on inside.
Learn more about pH electrodes and how to care for them.
Have more questions? Take a quick moment to get in touch with our expert sales engineers and technologists and we'll guide you to a solution.
Refex Combination Probes
Refex combination probes are available in industry standard sizes and designed to fit in any existing or new ‘socket’ where pH or ORP measurements are made. These sensors can be used in all applications, particularly those where fouling and drift are common, and have a usable lifetime five times longer than conventional sensors.
The Refex reference utilizes a patented, highly stable, non-porous polymeric interface instead of the traditional porous liquid junction as used by conventional reference electrodes. The active reference area comprises the entirety of the outside surface of the electrode. This extremely large contact area means that electrode performance is supremely resistant to coating. Poisoning effects such as drift and slow response are eliminated because the polymeric reference material is conductive to ions but not porous. The reference operates electrically, but electrolyte and process fluids are not exchanged.
Products in this product line currently include:
- 2001 Series 12mm pH Combination Probes
- 2001 Series NPT pH Combination Probes
- 2002 Series NPT Redox/ORP Combination Probes
- 2002 Series 12 mm Redox/ORP Combination Probes
What distinguishes Refex electrodes from competitors?
Refex electrodes represent a revolutionary technology advance in pH and ORP measurement. Refex reference junctions are manufactured from an electrochemically conductive, salt-loaded, polymeric matrix. When installed inline, there is no porous junction to allow liquid contact between process fluids and the internal (sealed for life) Ag/AgCl reference half-cell. Refex provides a stable mV output for measurement and ensures that process fluids do not poison or deplete reference cell electrodes or electrolyte.
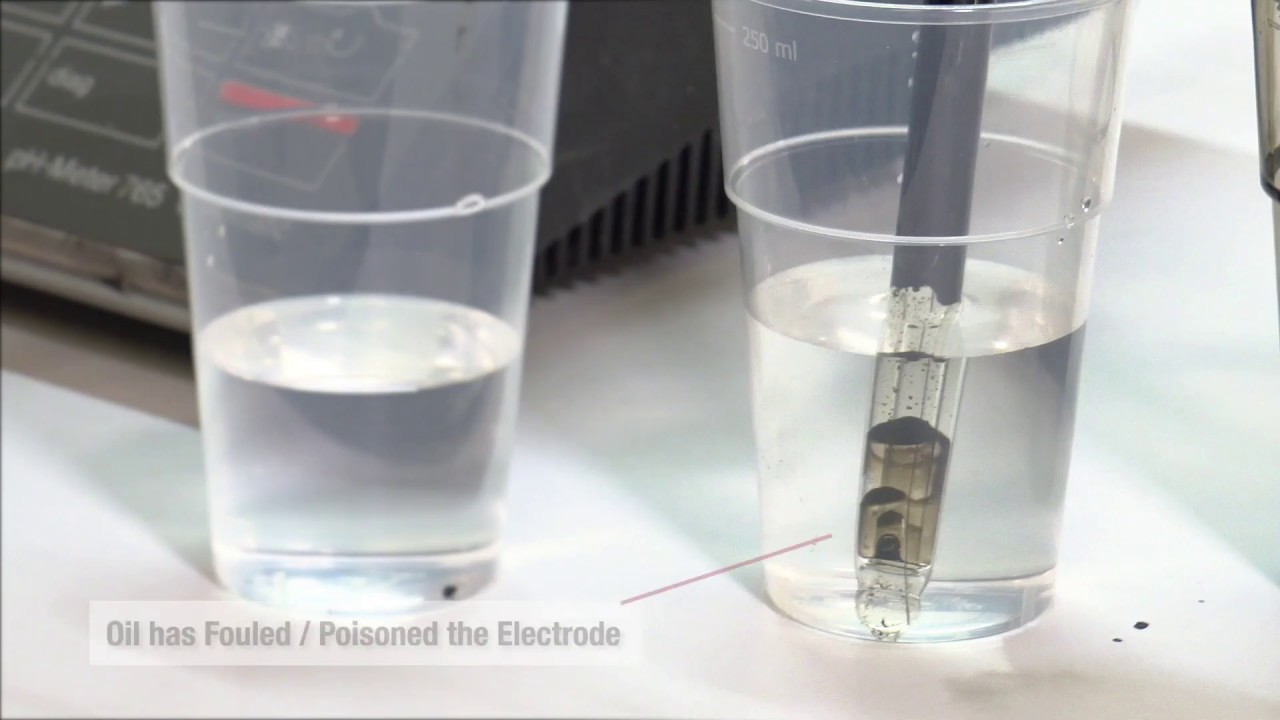
Refex electrodes continue to perform even when completely coated, as long as the coating remains wet and is conductive. Even when severe coating of the sensor inevitably slows electrode response, pH and ORP measurement remains accurate. Proven applications range from clean, cold river water to crude oil, sewage sludge and nasty chemical streams—all can be measured with a Refex reference interface.
Refex electrodes have a range of benefits, including:
- Ultra-low maintenance
- Lifespan five times longer than standard sensors
- Work in vacuum and high pressure (300 psi) conditions
- Never clog
- Never leak
- Provide faster response than traditional pH/ORP sensors.
Have more questions? Take a quick moment to get in touch with our expert sales engineers and technologists and we'll guide you to a solution.
Refex 12mm Separates pH, ORP and Reference Probes
Refex 12mm separates are available in all industry standard lengths and a variety of connector/integral cable styles to ensure they will fit in any 12mm ‘socket’ where pH or ORP measurement is made. Refex sensors work will all popular pH/ORP transmitters with high impedance inputs.
Refex reference sensors are a tremendous upgrade to traditional porous/flowing junction reference probes and can be used in all applications, especially those where fouling or poisoning conditions exist. With lifetimes expected to be at least 5 times longer than traditional designs, Refex reference electrodes represent significant savings in maintenance and replacement costs.
Refex 12mm sensors are available in a variety of packages to suit all pH and ORP applications, including pH glass sensors, platinum ORP sensors, and reference sensors. Currently, models in the product line include:
- 5610 Series 12mm pH Glass Electrodes
- 5710 Series 12mm Reference Probes
- 7610 Series 12mm Platinum Redox/ORP Electrodes
What applications are Refex pH and ORP electrodes typically used for?
Refex electrodes are exceptionally resistant to applications containing poisons such as cyanide, ammonia and sulfides. This is because the polymeric reference junction is non-porous and prevents process material from invading and contaminating the electrode internals, causing drift and inevitable failure.
Here are a few applications where Refex sensors make a real difference.
Have more questions? Take a quick moment to get in touch with our expert sales engineers and technologists and we'll guide you to a solution.
SOUTH FORK INSTRUMENTS IS COMMITTED TO SOLVING YOUR FACTORY AND PROCESS AUTOMATION CHALLENGES
Using effective communication and an in-depth understanding of your industry to develop practical solutions for your measurement needs.
SOUTH FORK INSTRUMENTS
3845 Buffalo Road
Auburn, CA 95602
Tel: (+1) 925-461-5059

 The traditional pH electrode features a glass tube with a small bulb at one end and an electrical wire or connection at the other. The tube may be a single tube (measurement electrode only) or may be a tube within a tube (combination sensor with both measurement and reference electrodes). The inside of the tube is filled with an electrolyte, usually a buffered chloride solution. The pH of the solution inside is usually neutral (a pH of roughly 7) but can vary depending upon the measurement application.
The traditional pH electrode features a glass tube with a small bulb at one end and an electrical wire or connection at the other. The tube may be a single tube (measurement electrode only) or may be a tube within a tube (combination sensor with both measurement and reference electrodes). The inside of the tube is filled with an electrolyte, usually a buffered chloride solution. The pH of the solution inside is usually neutral (a pH of roughly 7) but can vary depending upon the measurement application.
